Medical Device Security Basics: Protecting Patient Data
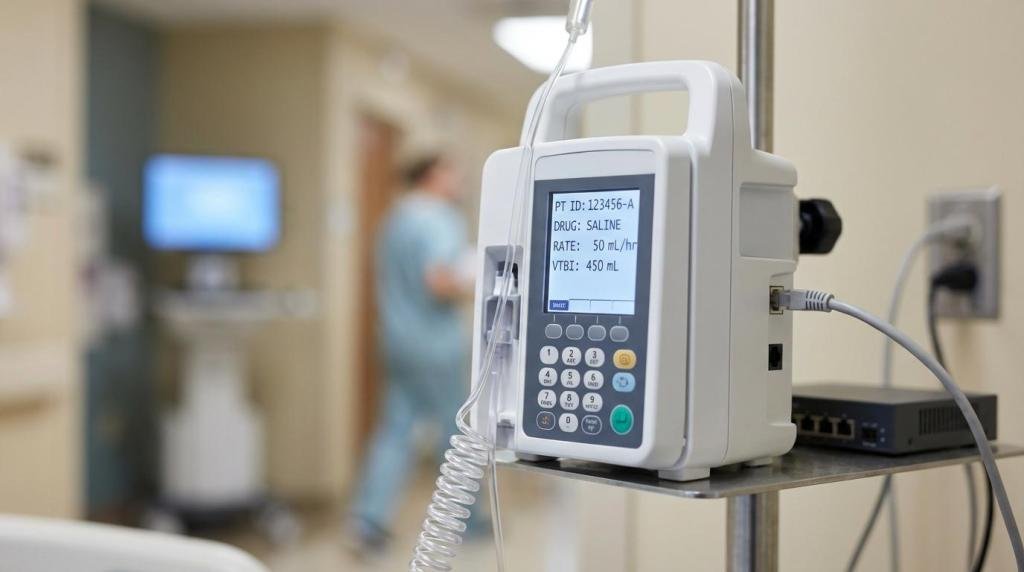
Network-connected medical devices sit at an uncomfortable intersection: they are computers on your network, they touch protected health information (PHI), and they support patient care where downtime is not just inconvenient, it can be dangerous.
For small to mid-sized healthcare organizations around Sacramento and Elk Grove, the challenge is rarely a lack of concern. It is the reality of mixed vendor ecosystems, long device lifecycles, and limited maintenance windows. The good news is that a strong baseline goes a long way when it is built around risk and clinical workflow, not generic IT assumptions.
Why medical devices require a different security mindset
A workstation that blue-screens is an IT ticket. A pump, monitor, imaging workstation, or lab analyzer that becomes unstable can interrupt care, delay results, or force a switch to manual processes under stress.
Medical devices also tend to be “owned” by multiple groups at once. IT manages networks and identity. Clinical engineering maintains devices and coordinates vendors. Clinical teams use devices continuously. If those groups are not working from the same playbook, security gaps show up as default credentials, open services, unknown devices on the network, or patches that never get scheduled.
One sentence that helps frame priorities: medical device security is patient safety plus data security, delivered through operational discipline.
The CIA triad, translated for clinical reality
The classic Confidentiality, Integrity, Availability (CIA) model maps cleanly to healthcare, but the stakes look different on a device fleet.
Confidentiality means PHI is protected on the device and as it moves across the network. HIPAA’s Security Rule technical safeguards center on access control, audit controls, integrity protections, and transmission security. Devices that store patient identifiers, test results, images, or logs need the same care you give servers and EHR-connected systems.
Integrity means data and device behavior can be trusted. If results, settings, or software can be altered, the risk is not only a breach; it can be a clinical error. This is where signed updates, secure boot concepts, and strong change control matter.
Availability is often the loudest requirement. Security controls that add friction in an emergency can be unacceptable. You still protect access, but you design it to match clinical urgency, including “break-glass” access with tight logging when appropriate.
A practical baseline for network-connected medical equipment
Start by treating devices as managed assets with known owners, known software versions, and known network dependencies. Without that, every other control becomes guesswork.
After you have an inventory and a way to classify risk, the baseline can be straightforward:
- Asset inventory and ownership
- Network segmentation by function and risk
- Strong authentication and removal of defaults
- Encryption where PHI or control traffic traverses shared networks
- Controlled remote access for vendors
- Patch and vulnerability management with clinical validation
- Central logging and alerting for abnormal behavior
- Documented incident response steps that include clinical engineering
A baseline is not “one size fits all.” An imaging workstation that stores studies has different exposure than a bedside device that only streams telemetry. The goal is consistency in how decisions get made.
A quick mapping of common issues to controls
| Common problem | What it can lead to | Practical control that helps |
|---|---|---|
| Unknown devices on the network | Unpatched systems, blind spots during incidents | Inventory plus network access control (NAC) or controlled onboarding |
| Default or shared credentials | Unauthorized access, lateral movement | Credential rotation, unique accounts, restricted admin access |
| Flat networks | One compromise spreads widely | VLAN segmentation, internal firewalls, allow-list rules |
| Legacy operating systems | Known exploits with no vendor patch | Isolation, compensating controls, replacement plan |
| Vendor remote tools with broad access | Data exposure, persistent access paths | Vendor access gateway, time-bound access, session logging |
| Unencrypted protocols for PHI | Interception, manipulation | TLS/IPsec where possible, secure tunnels between segments |
Network design that limits blast radius
Many device compromises become “big” because the environment is flat. Segmentation is the single most reliable way to reduce impact when a device has a vulnerability you cannot immediately fix.
A common pattern is to group devices by clinical function and risk, then tightly control what each segment can talk to. In practice, that means devices do not need broad internet access, and they rarely need to initiate connections to user networks.
After you’ve confirmed vendor requirements, segmentation rules often come down to “device talks only to the servers it must,” and everything else is blocked by default.
Once segmentation is in place, logging becomes more valuable. When a device that normally talks to one internal endpoint suddenly attempts DNS lookups to unknown domains or contacts an unfamiliar IP, it stands out quickly.
Identity, access, and configuration basics that prevent easy wins
Attackers love the simple stuff: default passwords, open management ports, and shared local accounts. Medical device fleets are unfortunately known for these issues, sometimes because of vendor defaults, sometimes because teams worry that changing settings could affect support.
You can reduce those risks without breaking clinical workflows by standardizing how devices are introduced and maintained. That includes a secure configuration checklist and clear coordination with the vendor and clinical engineering team.
A short set of controls that usually pays off early:
- Quick credential wins: Remove defaults, disable unused accounts, document admin ownership
- Service reduction: Turn off protocols and ports not required for operation or support
- Controlled remote support: No direct inbound access from the internet; broker it through a managed method with logging
- Time sync and logs: Accurate time is essential for investigations and HIPAA audit trails
Where multi-factor authentication (MFA) is feasible, use it for administrative actions and remote access. Where MFA could slow urgent care, focus on segmentation, strong device identity, and fast detection.
Patch and vulnerability management when you cannot patch on demand
Medical device patching is rarely as simple as “update Tuesday.” Vendors may require validation, devices may run specialized operating systems, and clinical windows can be tight. FDA guidance places strong expectations on cybersecurity as part of device safety, and newer requirements for “cyber devices” put more pressure on manufacturers to provide ongoing support and clearer documentation.
For healthcare organizations, the workable approach looks like this:
- Track what you own: model, firmware, OS, location, and criticality
- Subscribe to vendor bulletins and relevant advisories
- Triage vulnerabilities by clinical impact and exploitability, not just severity scores
- Test patches safely, ideally in a lab or on a representative device
- Schedule maintenance with clinical leadership, then document what changed
When a device is end-of-life or cannot be patched promptly, treat it like a risk acceptance with compensating controls: isolate it, restrict who can reach it, and monitor it closely.
Procurement and vendor accountability: ask for what you will need later
Device security is much easier when you bake expectations into purchasing and renewal cycles. Current FDA expectations have increased attention on Software Bills of Materials (SBOMs) and structured postmarket vulnerability handling, which helps customers track exposure when a library or chipset flaw is disclosed.
Put vendor security into the same category as warranty and uptime, because it affects both.
A few questions worth standardizing in your procurement checklist:
- SBOM availability: Can you provide an SBOM and keep it updated for the supported life of the device?
- Patch commitments: What is the expected timeline for security fixes and how are customers notified?
- Remote access method: What tools are used, how is access approved, and can sessions be logged?
- Data handling: What PHI is stored, for how long, and how is it encrypted at rest and in transit?
- End-of-support plan: What happens when the OS or device reaches end-of-life, and what are replacement paths?
These questions also make internal planning easier. If you know a device will stop receiving updates in three years, you can build replacement into budgeting instead of scrambling later.
Monitoring and incident response built for clinical environments
Security monitoring for devices works best when it is network-centered. Many devices cannot run traditional endpoint agents, and scanning can be risky if it disrupts operation. Passive discovery, protocol-aware monitoring, and careful log collection are common starting points.
Incident response should not be copied from a generic IT template. It needs a clinical “keep care going” track alongside the containment steps.
When teams run tabletop exercises, include clinical engineering and a representative clinical leader. The hard decisions happen at that intersection: whether to isolate a device, what backup devices exist, and how downtime procedures will be communicated.
A few events that should trigger immediate review:
- Unexpected outbound connections from a device segment
- Repeated authentication failures against device management interfaces
- A device that changes behavior after a vendor visit or update
- New devices appearing without a documented onboarding process
How a Sacramento-based managed IT partner typically supports device security
Many healthcare organizations do not need a separate “medical device security platform” to make progress. They need disciplined fundamentals, consistent monitoring, and a team that can coordinate across IT, clinical engineering, and leadership.
A SOC-led managed IT provider like Business PC Support commonly helps by hardening the surrounding environment: segmentation design, firewall policy, secure remote access, continuous monitoring, and incident response readiness. For healthcare organizations, that work often connects directly to HIPAA security requirements and to the broader ecosystem that includes EHR and EMR integrations.
Local presence matters during high-pressure events. When a device network issue is affecting operations, fast coordination with the people on site can be the difference between a controlled response and prolonged disruption.
A starting plan that works even in complex device environments
Begin with inventory, segmentation, and credential control, then add patch governance and monitoring as a repeatable process.
If you can answer these four questions with confidence, you are already ahead of most environments: What devices are connected, what they talk to, who administers them, and how you will respond when one behaves unexpectedly.